*IBP = Imaging Bulk Package
The individuals who appear are for illustrative purposes. All persons depicted are models and not real patients.
ISOVUE® (Iopamidol Injection) Imaging Bulk Package is the first FDA-approved contrast medium designed for multidose use—multiple single doses for multiple patients in the computed tomography suite and angiography procedures.
Sustainability
The Direct Result of Responsible Leadership
The Bracco approach to conserve iodine, reduce waste, lower costs
Bracco led the way in the industry by partnering with the FDA: Together we developed the IBP solution to help resolve workflow/resource challenges in the CT suite1
Using IBP is a compliant way to help:
- reduce costs
- waste less contrast
- save time
- individualize doses
Individualized dosing can reduce contrast waste and cost2

Personalized dosing using IBP is a recommended strategy for conserving iodine3
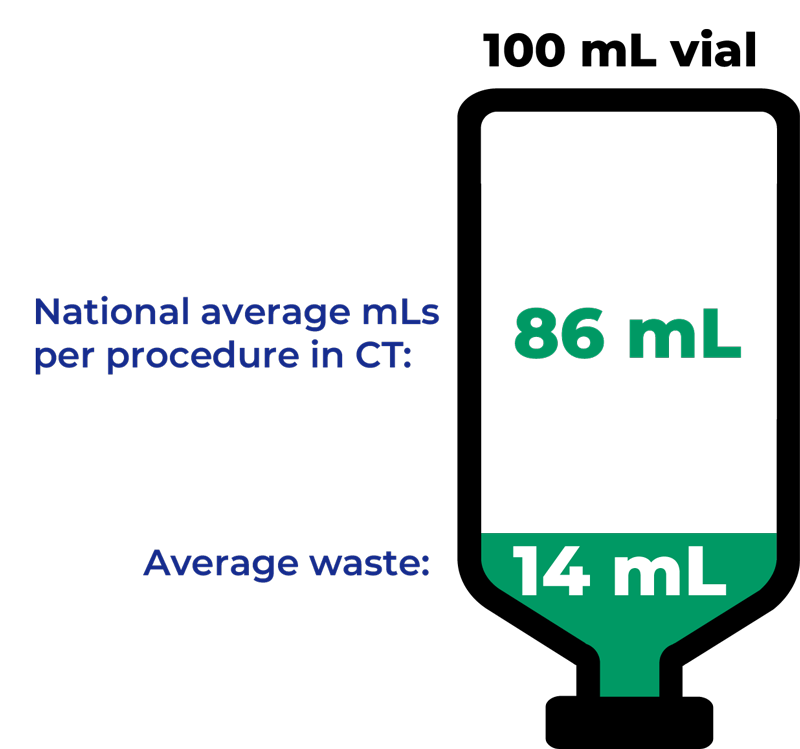
What Is Contrast Media Waste?
A common expense in the CT suite, contrast media waste is extra contrast fluid remaining in the vial which must be discarded after an individual dose is administered.
How does contrast waste add up?
- Average use: 86 mL of CT contrast per patient exam1
- Average of 14 mL of CT contrast would be “wasted” per 100 mL single dose vial (or pre-filled syringe)
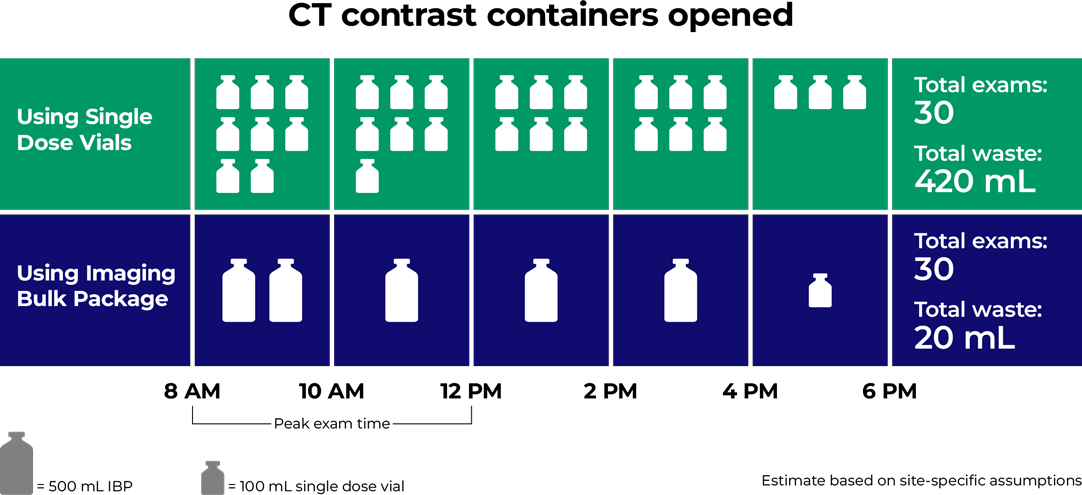
Eliminate waste from single-use containers
ISOVUE Imaging Bulk Package saves contrast and disposal costs associated with bottles4

Established Safety
ISOVUE products provide established efficacy and safety, 3,000+ published studies, and 40+ years of trusted use
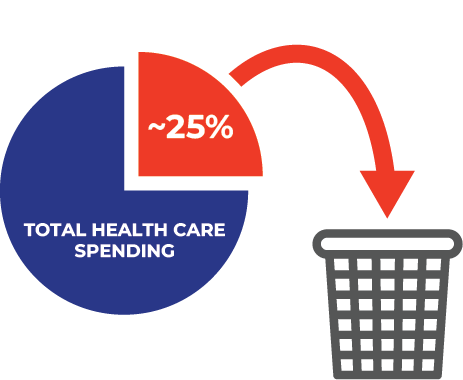
Increased Efficiency
Approximately 25% of total health care spending can be attributed to waste.5 Let us help you create the right intervention at the right time, for each patient.
Greater dosing efficiency results in less waste and improved cost savings1
- Accommodates multiple patients, procedures, and protocols6
- Streamlines processes by reducing time required for equipment setup
- More efficient than single-use vials and pre-filled syringes
- Simplified purchasing and inventory
- Fewer vials = less medical waste
IBP Product Features
Maximum use time
ISOVUE Imaging Bulk Package provides convenient dosing from the same container up to 10 hours after initial puncture to accommodate multiple patients.6*
*A maximum use time of 10 hours from initial closure entry is permitted to complete fluid transfer.
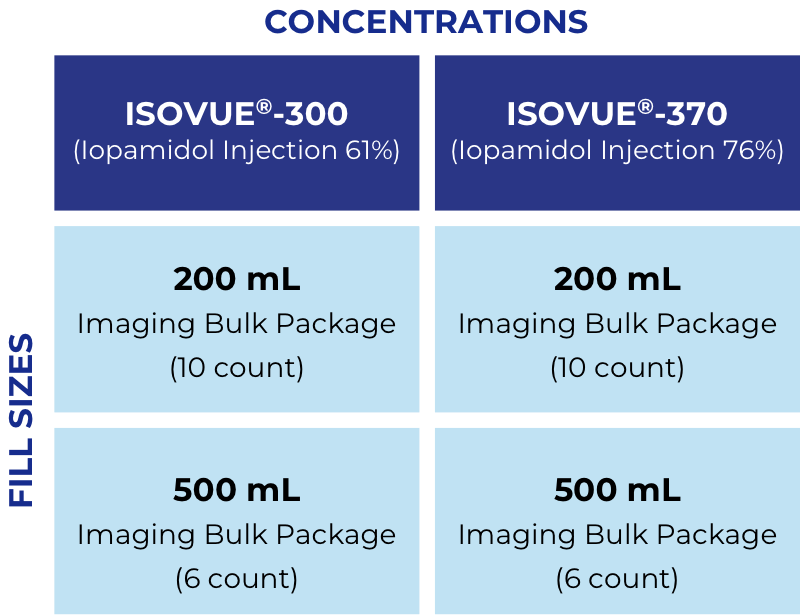
Flexible packaging options save time
Also available for cardiac catheterization

Connect with your Bracco partners
![]() IMPORTANT SAFETY INFORMATION
IMPORTANT SAFETY INFORMATION
ISOVUE-300, -370 Imaging Bulk Package is NOT FOR INTRATHECAL USE.
WARNINGS AND PRECAUTIONS | Severe Adverse Events-Inadvertent Intrathecal Administration
Serious adverse reactions including: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema have been reported due to the inadvertent intrathecal administrations of iodinated contrast media that are not indicated for intrathecal use.
INDICATION:
ISOVUE®-200, -250, -300, -370 (Iopamidol Injection) solution
ISOVUE®-300, -370 (Iopamidol Injection) solution Imaging Bulk Package is indicated for:
- angiography in adults throughout the cardiovascular system including cerebral and peripheral arteriography, coronary arteriography and ventriculography, selective visceral arteriography and aortography, peripheral venography (phlebography),
- in pediatric patients for angiocardiography
- in adult and pediatric intravenous excretory urography and contrast
- CT Head Imaging (to refine diagnostic precision in areas of the brain which may not have been satisfactorily visualized)
- CT Body Imaging (enhancement of computed tomographic images for detection and evaluation of lesions in the liver, pancreas, kidneys, aorta, mediastinum, abdominal cavity, pelvis and retroperitoneal space).
ISOVUE-300, -370 Imaging Bulk Package is for use only with an automated contrast injection system, contrast management system, or contrast media transfer set approved or cleared for use with ISOVUE-300, -370 Imaging Bulk Package.
ISOVUE-300, -370 Imaging Bulk Package is NOT FOR INTRATHECAL USE.
IMPORTANT SAFETY INFORMATION:
WARNINGS AND PRECAUTIONS
Severe Adverse Events-Inadvertent Intrathecal Administration Serious adverse reactions including: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema have been reported due to the inadvertent intrathecal administrations of iodinated contrast media that are not indicated for intrathecal use.
Special attention must be given to insure that this drug product is not inadvertently administered intrathecally.
Caution must be exercised in patients with severely impaired renal function, those with combined renal and hepatic disease, or anuria, particularly when larger or repeated doses are administered.
Clotting
Clotting has been reported when blood remains in contact with syringes containing nonionic contrast media. Serious, rarely fatal, thromboembolic events causing myocardial infarction and stroke have been reported during angiographic procedures with both ionic and nonionic contrast media, therefore meticulous angiographic techniques are recommended in addition to minimizing the length of the procedure to help decrease in vitro clotting.
Patients with Multiple Myeloma or Paraproteinemia
Radiopaque diagnostic contrast agents are potentially hazardous in patients with multiple myeloma or other paraproteinemia, particularly in those with therapeutically resistant anuria. The risk in myelomatous patients is not a contraindication; however, special precautions are required.
Patients with Sickle Cell Disease
Contrast media may promote sickling in individuals who are homozygous for sickle cell disease when injected intravenously or intraarterially.
Patients with Pheochromocytoma
Administration of radiopaque materials to patients known or suspected of having pheochromocytoma should be performed with extreme caution. If the possible benefits of such procedures outweigh the considered risks, the procedures may be performed; however, the amount of radiopaque medium injected should be kept to an absolute minimum. The blood pressure should be assessed throughout the procedure and measures for treatment of a hypertensive crisis should be available. These patients should be monitored very closely during contrast enhanced procedures.
Thyroid Storm
The use of iodinated radiopaque diagnostic agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule suggest that this additional risk be evaluated in such patients before use of any contrast medium.
Thyroid Dysfunction in Pediatric Patients 0 to 3 Years of Age
Hypothyroidism or transient thyroid suppression has been reported after both single and multiple exposures to iodinated contrast media.
Younger age, very low birth weight, prematurity, underlying medical conditions affecting thyroid function, admission to neonatal or pediatric intensive care units, and congenital cardiac conditions are associated with an increased risk of hypothyroidism after ICM exposure. Pediatric patients with congenital cardiac conditions may be at greatest risk given that they often require high doses of contrast during invasive cardiac procedures. An underactive thyroid during early life may be harmful for cognitive and neurological development and may require thyroid hormone replacement therapy. After exposure to ICM, individualize thyroid function monitoring based on underlying risk factors, especially in term and preterm neonates.
Severe Cutaneous Adverse Reactions
Severe Cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions.
Acute Renal Impairment / Failure
Diabetic nephropathy may predispose to acute renal impairment following intravascular contrast media administration. Acute renal impairment following contrast media administration may precipitate lactic acidosis in patients who are taking biguanides.
Preparatory dehydration is dangerous and may contribute to acute renal failure in patients with advanced vascular disease, diabetic patients, and in susceptible nondiabetic patients (often elderly with preexisting renal disease). Patients should be well hydrated prior to and following iopamidol administration.
Hypersensitivity / Anaphylaxis
Patients at increased risk include those with a history of a previous reaction to a contrast medium, with a known sensitivity to iodine, with a known clinical hypersensitivity (bronchial asthma, hay fever, and food allergies). A thorough medical history with emphasis on allergy and hypersensitivity, prior to the injection of any contrast medium, may be more accurate than pretesting in predicting potential adverse reactions. Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions in such patients should be considered.
Patients with Congestive Heart Failure
The potential transitory increase in the circulatory osmotic load in patients with congestive heart failure requires caution during injection. These patients should be observed for several hours following the procedure to detect delayed hemodynamic disturbances.
DRUG INTERACTION
Renal toxicity has been reported in a few patients with liver dysfunction who were given oral cholecystographic agents followed by intravascular contrast agents. Postpone administration of intravascular agents in any patient with known or suspected hepatic or biliary disorder who has recently received a cholecystographic contrast agent.
ADVERSE REACTIONS
The most frequent adverse reactions are: hot flashes, angina pectoris, flushing, bradycardia, hypotension, and hives.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1‑800‑FDA‑1088.
Please click here for full Prescribing Information for ISOVUE® products.
Please click here for full Prescribing Information for ISOVUE® Imaging Bulk Package products.
ISOVUE and ISOVUE Imaging Bulk Package are currently manufactured for Bracco Diagnostics
Inc. at three locations: BIPSO GmbH, Singen (Germany), Patheon Italia S.p.A., Ferentino (Italy), and
S. M. Farmaceutici SRL, Tito (Italy).
ISOVUE is a registered trademark of Bracco Diagnostics Inc.
IMPORTANT SAFETY INFORMATION | INDICATIONS AND USAGE
ISOVUE-300, -370 Imaging Bulk Package is NOT FOR INTRATHECAL USE.
WARNINGS AND PRECAUTIONS | Severe Adverse Events-Inadvertent Intrathecal Administration
Serious adverse reactions including: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema have been reported due to the inadvertent intrathecal administrations of iodinated contrast media that are not indicated for intrathecal use.
1. Spinazzi A, Marchildon PA, Murray EA, et al. Development of a New Imaging Bulk Package for Multipatient Use in MDCT. Am J Roentgenol. 2015 Oct;205(4):697-702.
2. Davenport MS, Parikh KR, Mayo-Smith WW, et al. Effect of Fixed-Volume and Weight-Based Dosing Regimens on the Cost and Volume of Administered Iodinated Contrast Material at Abdominal CT. J Am Coll Radiol. 2017 Mar;14(3):359-370.
3. Grist T, Canon CL, Fishman EK, et al. Short-, Mid-, and Long-term Strategies to Manage the Shortage of Iohexol. Radiology. 2022 Aug;304(2):246-492.
4. Data on file. Bracco Diagnostics Inc.; September 2021.
5. Shrank, W. H., Rogstad, T. L., & Parekh, N. Waste in the US health care system: estimated costs and potential for savings. JAMA. 2019 Oct;322(15), 1501-1509.
6. ISOVUE® (iopamidol injection) Imaging Bulk Package full Prescribing Information. Bracco Diagnostics Inc. April 2023.


